1 – Who should receive the booster dose against Covid-19 in 2024?
RESPONSE: PRIORITY GROUPS from 5 years of age and with greater vulnerability or conditions that increase the risk of severe forms of the disease. Therefore, these populations are recommended an annual dose (or every six months, for people aged 60 or over, immunocompromised and pregnant/postpartum women).
2 – Can people who belong to the priority group and have a history of different previous doses (D1, D2, D3, REF1, REF2) of vaccines against Covid-19 receive the annual booster in 2024?
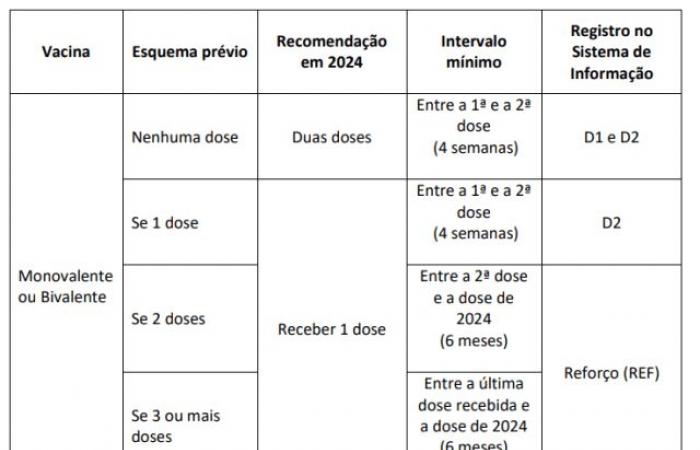
ANSWER: YES. Anyone in the priority group is eligible to receive a dose of the COVID-19 vaccine available in 2024. As described below:
If 1 dose: receive 1 dose after 4 weeks.
If 2 doses: receive 1 dose after 6 months.
If 3 or more doses: 1 dose after 6 months.
ATTENTION: A minimum interval of 6 months must be observed between the last dose received. For people aged 60 and over, immunocompromised and pregnant and postpartum women, a new dose is indicated in 2024 after an interval of 6 months. For other priority groups, the recommendation is ONE ANNUAL DOSE.
3 – Can people who belong to the priority group and have never been vaccinated (no dose) receive the annual booster in 2024?
ANSWER: YES. People in the priority group who have never been vaccinated (NO DOSE) must receive two doses of the COVID-19 vaccine (monovalent or bivalent) with an interval of 4 weeks between the two doses (primary schedule) and end the 2024 schedule.
ATTENTION: Pregnant women, postpartum women, immunocompromised people and elderly people aged 60 or over, in the situation described above, in addition to the TWO DOSES, should receive a booster dose with the bivalent vaccine or with the most up-to-date vaccine available, this can be carried out 6 months after the last dose .
4 – Can immunocompromised people who belong to the priority group and have never been vaccinated (no dose) receive the annual booster in 2024?
ANSWER: YES. Immunocompromised people who have never been vaccinated (NO DOSE) MUST receive three doses of the COVID-19 vaccine (monovalent or bivalent) with an interval of 4 weeks between the first and second dose, and 8 weeks between the second and third dose (primary schedule of the immunocompromised). A booster dose of a bivalent vaccine or the most up-to-date vaccine available can be administered 6 months after the last dose of this regimen.
5 – What are the priority groups?
ANSWER: People aged 60 and over; People living in long-term care institutions (ILPI and RI), and their workers; Immunocompromised people from 5 years of age; Indigenous people (from 5 years of age); Ribeirinhos (from 5 years of age); Quilombolas (from 5 years of age); Pregnant and postpartum women; Health workers; People with permanent disabilities (from 5 years of age); People with comorbidities (from 5 years of age); Persons deprived of liberty (≥ 18 years old); Employees of the deprivation of liberty system; Adolescents and young people complying with socio-educational measures; and Homeless people.
6 – Which vaccine is recommended for a booster dose for priority groups in 2024?
RESPONSE:
Age group 5 to 11 years – Pfizer COVID-19 Vaccine (5 to 11 years) – orange cap bottle
Age group from 12 years of age – Bivalent COVID-19 vaccine (bivalent Comirnaty vaccine) – Pfizer (gray cap bottle)
7 – Can people who do not belong to priority groups and do not have a primary schedule (two doses) get vaccinated in 2024?
ANSWER: YES. It should be noted that primary vaccination schedules against Covid-19 will no longer be routinely recommended for people aged 5 years or older who are not part of the priority group. However, if an individual who has not been previously vaccinated (no previous dose) or who has only received one dose of the vaccine against Covid-19, chooses to be vaccinated, they MAY initiate and/or
complete the primary vaccination schedule, consisting of TWO DOSES with an interval of 4 weeks between doses.
7.1 – Which vaccine can be used in this case?
RESPONSE: The available COVID-19 vaccine (monovalent or bivalent) and recommended for age must be used, with a minimum interval of 4 weeks between doses.
8 – Can people aged 5 or over who DO NOT belong to the priority groups and already have a primary schedule (two doses) receive the booster dose in 2024?
ANSWER: NO. As at this time there is no indication of new doses. Based on the WHO recommendation, the PNI’s guidance for the vaccination strategy against covid-19 in 2024 is to receive a booster dose for groups at greater risk of worsening the disease.
9 – Has the covid-19 vaccine for children aged 6 months to under 5 years of age become part of the routine National Vaccination Calendar?
ANSWER: YES. Licensed COVID-19 vaccines that are indicated for children and acquired by the National Immunization Program (PNI) will be part of the National Vaccination Calendar for children from January 1, 2024 (Technical Note no. 118/2023- CGICI/ DPNI/SVSA/MS). Therefore, the entire population between 6 months and 4 years, 11 months and 29 days NOT vaccinated or with an incomplete vaccination schedule according to age group, is eligible for routine vaccination in the National Childhood Vaccination Calendar.
10 – Do children aged 6 months to 4 years, 11 months and 29 days, with a complete schedule (three doses), need to receive additional doses in 2024?
ANSWER: NO. The vaccination schedule for this public is considered complete with receipt of THREE DOSES of the COVID-19 vaccine, with NO new doses being necessary, so far.
11 – For children who do NOT belong to the priority group and who have started the recommended vaccination schedule for the age group of 6 months to 4 years, 11 months and 29 days, with the Pfizer vaccine (wine cap bottle) and have reached 5 years of age without Having received all three doses, what is the recommendation?
RESPONSE: Complete the TWO DOSE schedule with the Pfizer immunizer (orange cap bottle) and the recommended interval for the age group of 5 to 11 years, 11 months and 29 days (4 weeks between the first and second dose), and consider the schedule finished.
11.1 – In the situation above, if the child belongs to the priority group, what is the recommendation?
ANSWER: Complete the two-dose schedule with the immunizing agent and recommended interval (4 weeks between the first and second dose). If immunocompromised, pregnant and postpartum women, they should receive a booster dose with the vaccine indicated for their age group, 6 months after the last dose of this schedule.
ATTENTION: Immunocompromised people who are NOT vaccinated MUST receive a three-dose schedule of the COVID-19 vaccine (monovalent or bivalent) with an interval of 4 weeks between the first and second dose, and 8 weeks between the second and third dose (primary schedule for the immunocompromised).
12 – What is the recommended vaccine for children?
RESPONSE:
Pfizer Pediatric Vaccine (mRNA) – wine-capped vial:
The Pfizer Pediatric vaccine (RNAm) will be used routinely. The recommended age for vaccination is: first dose at 6 months, second dose at 7 months and third dose at 9 months of age. All children between 6 months and 4 years, 11 months and 29 days can receive three doses (1st DOSE + 2nd DOSE + 3rd DOSE) of the Pfizer COVID-19 immunizer (wine cap bottle). The recommended interval is 4 weeks between the first and second
doses and 8 weeks between the second and third doses. (Technical Note nº 118/2023- CGICI/DPNI/SVSA/MS).
CoronaVac vaccine (inactivated):
The CoronaVac vaccine can be used in this population in specific situations, such as: rescuing children not vaccinated at the recommended age, lack of the recommended immunizer in the locality or contraindications to pediatric Pfizer in children aged 3 and 4 years old.
13 – Regarding insertion in information systems, what is the recommendation?
RESPONSE:
Vaccination Calendar (routine):
VACCINE ROOMS OF REFERENCE CENTERS FOR SPECIAL IMMUNOBIOLOGICALS (CREATE): The Reference Centers for Special Immunobiologicals – CRIEs must register the applied doses of immunobiologicals indicated for people with special clinical conditions in the SIPNI, in the SPECIAL strategy.
VACCINE ROOMS IN HOSPITALS, MATERNITIES AND POLYCLINICS: Hospitals, maternity wards and polyclinics with vaccination services must register the doses administered in SIPNI.
VACCINE ROOMS WITH OWN SYSTEMS: Hospitals, maternity wards and polyclinics with vaccination services that use their own systems will be able to record the doses applied in SIPNI or integrate the system with the National Health Data Network – RNDS through the DATASUS Services Portal, https://servicos- datasus.saude.gov.br/
INDIGENOUS HEALTH SERVICES VACCINE ROOMS: Records of doses of immunobiologicals applied in Indigenous Health Services must be made at SIPNI.
PRIMARY HEALTH CARE VACCINE ROOMS – APS: Vaccines administered in APS services will continue to be recorded with doses applied in the e-SUS APS applications (vaccination module in the Electronic Citizen Record – the PEC, in the Simplified Data Collection module – the CDS – and in the e-application SUS Vaccination) for more information, consult the eSUS APS manual and support materials available on the website:
https://sisaps.saude.gov.br/esus/
Campaign:
ALL VACCINE ROOMS: Use SIPNI or any other system integrated with the National Health Data Network – RNDS through the DATASUS Service Portal, https://servicos-datasus.saude.gov.br/
14 – Can vaccine interchangeability be carried out in the primary schedule?
RESPONSE: The primary regimen must be carried out, preferably, with the same immunizer. In case of unavailability or discontinuation of the vaccine initially used, the vaccine from another manufacturer or vaccine platform may be used to complete the regimens. The dose should be administered within the interval
previously scheduled, respecting the interval adopted for the immunizing agent used in the first dose.
15 – Children aged 6 months to under 5 years of age, who started the three-dose schedule and did not complete it within the age range (they turned 5 years old before finishing the schedule)?
RESPONSE:
If 1 dose before age 5: receive 1 dose and end the schedule.
If 2 doses before age 5: end schedule.
If 3 doses before age 5: consider complete schedule (NO NEED TO RECEIVE NEW DOSES)
16 – VACCINATION FOR TRAVELERS?
RESPONSE: If traveling, the requirements of the destination country must be checked. If the country requires a vaccination schedule, and the individual DOES NOT have any doses, they may receive a schedule of up to two doses. States and municipalities will be able to evaluate situations individually with the aim of finding the best vaccination schedule, according to the availability of the vaccine and the requirements of the destination countries, which guarantees protection and safety for the individual.
Main questions_Covid-19 vaccination strategy in 2024 (1)
Like this:
Like Loading…