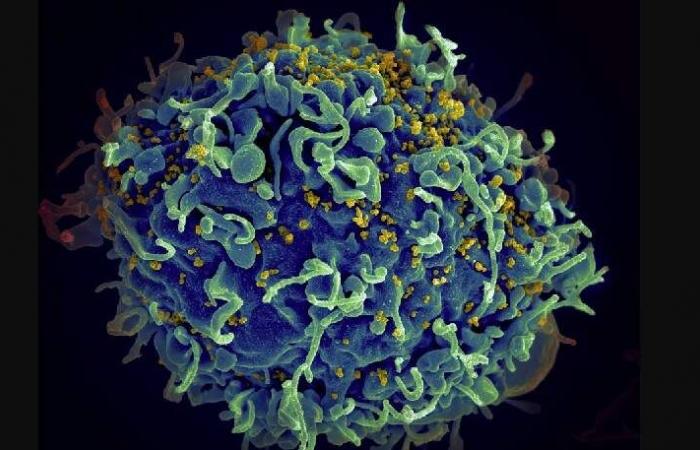
Image shows the HIV virus (in yellow) infecting a human cell. (Photo: National Cancer Institute/Unsplash)
The Ministry of Health has included a new group for the HPV vaccine and will incorporate a medicine for patients living with HIV. Since the 22nd, patients with recurrent respiratory papillomatosis (RRP) are now part of the priority group to receive the HPV vaccine.
According to the ministry, this decision was motivated by studies showing the benefits of the vaccine as a complementary treatment, significantly reducing the return of the disease in vaccinated patients.
This is because RRP is a rare condition caused by HPV itself. The clinical picture is characterized by warts in the respiratory tract. Surgical treatment is common, but recurrences are frequent and can be serious, especially in children.
Therefore, since 2006, the HPV vaccine has been considered as part of treatment, with encouraging results. The vaccine will be available with a doctor’s prescription and, for children under 18, parental consent is required.
Recently, the ministry also announced that the vaccine against HPV, a virus associated with more than 90% of cervical cancer cases, will be administered in a single dose in the Unified Health System (SUS).
The recommendation is for a specific audience: children and teenagers aged 9 to 14. Immunosuppressed people and victims of sexual violence, who can also receive the vaccine on the public network, will continue with the previous schedule (up to three doses).
HIV medication
The new medicine for the treatment of patients with HIV is Fostensavir trometamol (600mg), which will be available in the Unified Health System (SUS) for adult patients who face multiple resistance to conventional treatments against the virus.
The National Commission for the Incorporation of Technologies into the Unified Health System (Conitec) approved the incorporation of the medicine in March, but only now a decree must be published. After that, the medicine has up to 180 days to be available on the SUS.
Also according to the ministry, the criteria for expanding the public covered by the new treatment model could be reviewed in six months, observing, for example, the growth trend in prescriptions and the availability of the medicine in stock on the network.
At the time of approval by Conitec, the Ministry of Health stated that the inclusion of this new medication is in line with the ministry’s objective of promoting a better quality of life for those living with the AIDS virus, as people who develop resistance to multiple medications face a higher risk of contracting opportunistic diseases.
The last time a medication for multidrug-resistant people was included in the SUS was eight years ago, when 200mg etravirine was incorporated. The information is from G1.
Tags: Ministry Health includes group HPV vaccine incorporates medicine HIV patients